Shuo Yang, et al
[Abstract]
Treatment strategies for advanced non–small cell lung cancer (NSCLC) patients who develop oligo-residual disease (ORD) after responding to immunotherapy remain undefined. The BOOSTER study is the first randomized, phase II trial to evaluate whether adding local ablation to ongoing anti–PD-1/PD-L1 therapy improves clinical outcomes.
A total of 108 patients with advanced NSCLC and ORD were randomized to receive either ablation plus continued immunotherapy or immunotherapy alone. Ablation modalities included cryoablation and thermal ablation.
The primary endpoint of progression-free survival was significantly improved in the ablation arm. Subgroup analysis showed that cryoablation (performed using Hygea’s cryoablation system) achieved the longest progression-free survival among ablation modalities.
Key Results
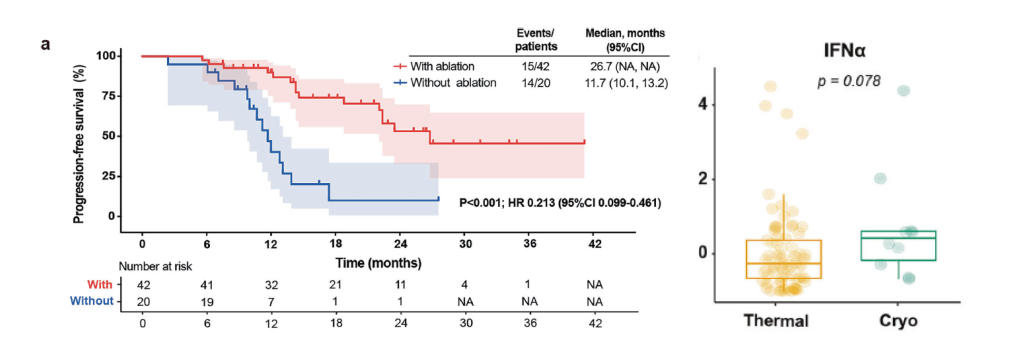
• Median Progression-Free Survival (PFS): 26.7 months (ablation + immunotherapy) vs. 11.7 months (immunotherapy alone) p < 0.001
• Systemic IFN-α Increase: Observed after cryoablation, indicating a distinct immune effect
• Safety: One grade 3 pneumothorax in the ablation + immunotherapy group; overall good tolerability
• Reduced Systemic Progression: Lower rate of out-of-field progression in the ablation group
These findings suggest that cryoablation, when combined with continued anti–PD-1/PD-L1 therapy, may enhance both local control and systemic antitumor immunity in advanced NSCLC with oligo-residual disease.
