GUAN Haitao, FAN Zeyang, WANG Jian, TONG Xiaoqiang, ZOU Yinghua*
(Department of Interventional Radiology and Vascular Surgery, Peking University First Hospital, Beijing 100034, China)
[Abstract] Objective To observe the efficacy and safety of CT-guided percutaneous combined cryoablation for malignant lung tumors. Methods Data of 22 patients with malignant lung tumors who received CT-guided percutaneous combined cryoablation and hyperthermia were retrospectively analyzed. According to the size, shape and location of the lesions, radical ablations were performed in 15 cases, while palliative ablations were performed in 7 cases. Adverse reactions and complications during operation and within 4 weeks after operation were observed. Enhanced CT scan of the chest was reviewed at 1, 3 and 6 months after treatment, and the local ablation efficacy was evaluated using modified-response evaluation criteria in solid tumors (mRECIST). Results All ablation were successfully accomplished in 22 patients without obvious intraoperative adverse effects. Slight pneumothorax or hydropneumothorax occurred in 6 cases during and immediately after operation, and then recovered without intervention. Local skin frostbite was noticed in 2 cases during operation, which recovered after local treatment. One case suffered from delayed massive pneumothorax at 1 week after operation and relieved through thoracic drainage. No other complications occurred. At 1, 3 and 6 months after operation, among 15 patients who underwent radical ablation, complete response was observed in 14 (14/15, 93.33%), 14 (14/15, 93.33%) and 13 (13/15, 86.67%) cases, respectively, while among 7 cases who underwent palliative ablation, partial response was found in 7 (7/7, 100%), 6 (6/7, 85.71%) and 4 (4/7, 57.14%) cases, respectively. Conclusion CT-guided combined cryoablation and hyperthermia for malignant lung tumors had definite short-term local effect and controllable safety.
[Keywords] lung neoplasms; cryoablation; tomography, X-ray computed
[CLC No.] R734.2; R815 | [Document code] A | [Article ID] 1672-8475(2023)01-0036-04 |
DOI: 10.13929/j.issn 1672-8475. 2023.01.009
Primary lung cancer and metastatic lung cancer are common in clinical practice [1], which seriously endangers the health of patients. The treatment options of middle and advanced lung cancer are limited. Therefore, adopting reasonable and effective treatment option depending on the different stages of the disease and the general conditions of patients to prolong the survival period and improve the quality of life of patients is the direction of clinical research and efforts [2]. In recent years, the combination of local treatment with systemic treatment has been used for the treatment of middle and advanced lung cancer that cannot be surgically resected. The radiofrequency ablation, microwave ablation and cryoablation have accurate local inactivation and control effect, and can achieve radical curative effect for some early lesions. After treatment, the tumor antigen exposure may enhance the body's anti-tumor immune response, thus having a synergistic effect with systemic therapeutic drugs [3]. Cryoablation is one of the important options of local treatment [4]. Cryoablation represented by argon-helium knife, which achieves the anti-tumor purpose relying on ultra-low temperature, has been used in the treatment of a variety of solid tumors. The combined cryoablation and hyperthermia system (Combo knife), which uses liquid nitrogen as the refrigerant, treats tumors relying on a combination of deep cryoablation with hyperthermia. This study was performed to observe the local efficacy and safety of CT-guided percutaneous combined cryoablation and hyperthermia in the treatment of malignant lung tumors.
1.1 General data: A retrospective analysis was performed on 22 patients with lung malignant tumor who underwent CT-guided percutaneous combined cryoablation and hyperthermia in Peking University First Hospital from September 2018 to October 2020, including 16 males and 6 females, aged 35 - 80 years old, with median age of 64 years old; including 8 patients with single lesion and 14 patients with multiple lesions (the largest lesion was selected as the target lesion); including 19 patients with primary lung cancer (3 patients with squamous cell carcinoma, 14 patients with adenocarcinoma, 2 patients with small cell carcinoma) and 3 patients with metastatic lung cancer (2 patients with liver cancer, the primary lesion, and 1 patient with osteosarcoma of humerus). The long diameter of the lesion was 13.26~100.31 mm, with median long diameter of 33.92 mm, and the short diameter was 13.15~86.50 mm, with median short diameter of 28.14 mm. 15 patients underwent radical ablation (maximum diameter of the target lesion: ≤ 5 cm, with clear boundary, distance from the heart, large blood vessels and other important organs: > 1cm), and 7 patients underwent palliative ablation (maximum diameter of the target lesion: > 5 cm, with unclear boundary and irregular shape, or close to important parts such as hilus pulmonis and mediastinum, complete ablation not expected).
Inclusion criteria: ① Preoperative diagnosis was confirmed by imaging and pathological examination, and radical or palliative ablation was performed according to the size, shape and location of the lesion.② There was no indication of ablation or the patient was reluctant to undergo ablation. The severe cardiopulmonary dysfunction and coagulation dysfunction were excluded. Before operation, patients or their families signed the informed consent.
1.2 Instruments and methods: GE 750HD CT instrument was used as the guiding device. The ablation device includes the co-ablation system (Combo knife) and the disposable sterile cryoablation needle (14G, 15 cm or 18 cm in length). The inserting site, path and number of needles were set according to preoperative imaging data. The ablation needle was tested in vitro before puncture to ensure it was under normal condition. The patient position was selected appropriately according to the lesion site, and the skin puncture point was determined through CT scan. After routine disinfection, 2% lidocaine was injected with a 5ml syringe for local anesthesia, while the syringe needle was retained. CT scan was performed again to observe the inserting angle and length with the needle as reference. A small incision was made with a scalpel at the puncture point and the lesion was punctured with the ablation needle, while the inserting angle and depth were adjusted if necessary. After the inserting site was confirmed satisfactory through CT scan, the co-ablation system (Combo knife) was connected and the ablation was performed in two cycles, that is, rewarming for 5 - 8 min after cryoablation for 15 min, with an interval of 5 - 10 min. During this period, the position of ablation needle and the size of the ice ball were observed through CT scan. If necessary, the position of ablation needle was adjusted. Then, cryoablation was performed for 10 - 15 minutes, and rewarming was performed for 5 min. In case of radical ablation, 5 - 10 mm beyond the lesion edge by the ablation zone was deemed as the endpoint of therapy. In case of palliative ablation, the ablation zone should be extended as much as possible according to the location of the lesion and the patient's tolerance. After ablation, the ablation needle was removed and the lung-wide CT scan was performed to observe for any complications such as bleeding and pneumothorax.
Symptomatic support treatment was performed after ablation. After radical ablation, in case of residue or progression of the target lesion, combined cryoablation and hyperthermia or a combination with systemic therapy may be performed. Palliative ablation should be followed by systemic therapy, or, depending on pathological results, chemotherapy, targeted therapy and immunotherapy.
1.3 Observation indexes and follow-up: The adverse reactions and complications were observed during ablation and within 4 weeks after ablation. The enhanced CT scan of the chest was
reviewed at 1, 3, and 6 months after ablation. The efficacy was evaluated according to the modified-response evaluation criteria in solid tumors (mRECIST), including complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD).
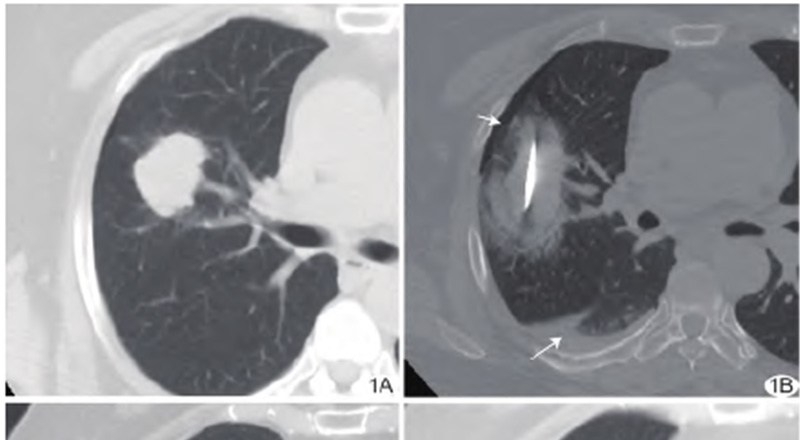
Figure 1 A 64-year-old female patient with adenocarcinoma of upper lobe of right lung underwent radical combined cryoablation and hyperthermia. A. Preoperative enhanced axial CT scan of the chest showed a 2.9 cm × 4.4 cm lesion in the upper lobe of right lung, which was visibly enhanced. B. Intraoperative plain CT scan of the chest showed that the lesion was fully covered by the ablation zone, and "halo sign", slight pneumothorax (short arrow) and pleural effusion (long arrow) were observed; C. At 1 month after ablation, the enhanced axial CT scan of the chest showed no enhancement of the ablation lesion, with regular edges and visible cavity in the lesion, and an efficacy evaluated as CR. D. At 6 months after ablation, the enhanced axial CT scan of the chest showed further absorption reduction of the ablation lesion, with an efficacy evaluated as CR.
All 22 patients completed the ablation successfully. 16 patients (16/22, 72.73%) underwent single-needle ablation, and 6 patients (6/22, 27.27%) underwent double-needle ablation. No significant adverse reactions were observed during ablation. Slight pneumothorax or hydropneumothorax was observed in 6 patients (6/22, 27.27%) during and immediately after ablation, which improved spontaneously without intervention. Mild frostbite in local skin was observed in 2 patients (2/22, 9.09%) during ablation, which was relieved after local treatment. Delayed massive pneumothorax was observed in 1 patient (1/22, 4.55%) at 1 week after ablation, which was recovered after closed thoracic drainage. No other complications were observed.
At 1 month after radical ablation, there were 14 patients with CR (14/15, 93.33%) and 1 patient with PR (1/15, 6.67%, with local residual, underwent the radical combined cryoablation and hyperthermia again). At 3 months after ablation, there were 14 patients with CR (14/15, 93.33%) and 1 patient with PD (1/15, 6.67%, with local recurrence, underwent the radical combined cryoablation and hyperthermia again). At 6 months after ablation, there were 13 patients with CR (13/15, 86.67%) and 2 patients with PD (2/15, 13.33%, underwent systemic treatment). Refer to
At 1 month after palliative ablation, there were 7 patients with PR (7/7, 100%). At 3 months after ablation, there were 6 patients with PR (6/7, 85.71%) and 1 patient with SD (1/7, 14.29%). At 6 months after ablation, there were 4 patients with PR (4/7, 57.14%), 2 patients with SD (2/7, 28.57%), and 1 patient with PD (1/7, 14.29%).
As one of the locally minimally invasive treatment options, cryoablation has been widely applied in the treatment of lung tumors by virtue of its less injury, accurate location, accurate efficacy, repeatability, and requiring for no general anesthesia [5-7]. For early lesions with maximum diameter ≤ 3 cm, after cryoablation, the local control rate is higher and the local progression rate is lower [8-9]. For middle and advanced malignant tumors with no indications of surgical resection, such as large and multiple lesions and metastatic cancers, cryoablation can also be used as a palliative treatment to achieve cytoreduction, relieve symptoms and activate tumor immune response. It has become an important part of the combination of chemotherapy, targeted therapy and immunotherapy [10-11]. With liquid nitrogen as the refrigerant, the co-ablation system (Combo knife) can achieve a more ideal destruction of tumor tissue in the alternating process of cryoablation and hyperthermia, with relatively stable ablation zone. In recent years, its value in the treatment of lung tumors has been recognized [12-13].
When CT-guided percutaneous combined cryoablation and hyperthermia radical ablation is performed with co-ablation system (Combo knife), the key to ensure the efficacy is to make the ablation zone fully cover the lesion. Currently, it is believed that the ablation zone should be at least 5-10 mm beyond the lesion edge to effectively reduce the local recurrence [8]. Compared with other hyperthermia options, the range of ice balls of cryoablation is not completely consistent with the range of tumor. Moreover, the lung is an aerated tissue, and its cold and heat conduction is different from that of solid tissue. Therefore, it is recommended to appropriately extend the zone of cryoablation. Due to the influence of the working range of the ablation needle, and the size, shape and location of the lesion, some lesions cannot reach the ideal safety boundary. In such case, multi-needle layout and prolonged ablation time may be considered to make up for it. Fully getting familiar with the operating characteristics of the device and the characteristics of tumors in different sites of the lung is of great significance to achieve the optimal efficacy. In this study, radical ablation (15 patients) and palliative ablation (7 patients) were performed on lung cancer lesions depending on the size, shape and location of such lesions. Among 15 patients, at 1 month after radical ablation, there were 14 patients with CR and 1 patient with PR. At 3 months after ablation, there were 14 patients with CR and 1 patient with PD, which were considered to be related to insufficient ablation zone. In 7 patients, tumor necrosis was clear within the short-term ablation zone after palliative ablation, and cytoreduction was achieved. At 3 and 6 months after ablation, residual tumor progression required a combination with systemic therapy.
The common complications of cryoablation include bleeding, pneumothorax, infection and skin injury, and the rare complication is cold shock. The incidence of complications is affected by the location of the lesion, basic lung conditions (such as severe emphysema, pulmonary fibrosis, etc.) and operator’s proficiency [14]. In this group, slight pneumothorax or hydropneumothorax was observed in 6 patients during and immediately after ablation, which improved spontaneously and was considered to be related to the thicker ablation needle and the relatively long treatment time. Delayed massive pneumothorax was observed in 1 patient at 1 week after ablation, with significant dyspnea symptom, which was radiofrequency ablation, microwave ablation and recovered after closed thoracic drainage. Frostbite on chest wall was observed in 1 patient and frostbite on local thigh skin was observed in 1 patient, which was related to insufficient protection of cryogenic pipeline during ablation and alleviated after locally topical medication. This suggests that sufficient isolation of skin, avoiding contact with device pipeline and close observation during ablation are helpful to avoid skin frostbite. No complications such as severe bleeding and infection were observed.
In conclusion, CT-guided percutaneous combined cryoablation and hyperthermia had accurate short-term local effect in the treatment of malignant lung tumors, with controllable safety. However, the sample size of this study was small, and some patients received systemic therapy at the same time. Therefore, the sample size should be expanded for long-term follow-up and further observation of long-term efficacy.
Reference
[1] BADE B C, DELA CRUZ C S. Lung cancer 2020: Epidemiology, etiology, and prevention[J]. Clin Chest Med, 2020 ,41(1):1-24.
[2] ZHAO Y, ADJEI A A. New strategies to develop new medications for lung cancer and metastasis[J]. Cancer Metastasis Rev, 2015,34(2):265-275.
[3] KATZMAN D, WU S, STERMAN D H. Immunological aspects of cryoablation of non-small cell lung cancer: A comprehensive review [J]. J Thorac Oncol, 2018,13(5):624-635.
[4] ZHANG Y S, NIU L Z, ZHAN K, et al. Percutaneous imaging-guided cryoablation for lung cancer [J]. J Thorac Dis, 2016, 8 (Suppl 9): S705-S709.
[5] NOMORI H, YAMAZAKI I, SHIRAISHI A, et al. Cryoablation for T1N0M0 non-small cell lung cancer using liquid nitrogen[J]. Eur J Radiol, 2020, 133:109334.
[6] DU AN H, ZHENG S Y, LUO C, et al. Cryoablation for advanced non-small cell lung cancer: A protocol for a systematic review[J]. BMJ Open, 2020, 10(9):e033460.
[7] GAO W, GUO Z, SHU S, et al. The application effect of percutaneous cryoablation for the stage III B/IV advanced non-small-cell lung cancer after the failure of chemoradiotherapy[J]. Asian J Surg, 2018, 41(6):530-536.
[8] YASHIRO H, NAKATSUKA S, INOUE M, et al. Factors affecting local progression after percutaneous cryoablation of lung tumors [J]. J Vase Interv Radiol, 2013, 24 (6):813-821.
[9] ZHANG X, TIAN J, ZHAO L, et al. CT-guided conformal cryoablation for peripheral NSCLC: Initial experience [J]. Eur J Radiol, 2012, 81(11):3354-3362.
[10] LIN M, LIANG S Z, WANG X H, et al. Clinical efficacy of percutaneous cryoablation combined with allogenic NK cell immunotherapy for advanced non-small cell lung cancer [J]. Immunol Res, 2017, 65(4):880-887.
[11] FENG J, GUIYU D, XIONGWEN W. The clinical efficacy of argon-helium knife cryoablation combined with nivolumab in the treatment of advanced non-small cell lung cancer [J]. Cryobiology, 2021, 102:92-96.
[12] 魏颖恬,肖越勇,亚洲冷冻治疗学会.影像学引导肺癌冷冻消融治疗专家共识2018版[J].中国介入影像与治疗学,2018,15(5):259-263.
[13] 中国抗癌协会肿瘤介入学专业委员会,中国医师协会介入医师分会,中国临床肿瘤学会放射介入治疗专家委员会,等.冷热多模态消融治疗肝脏恶性肿瘤操作规范专家共识[J].中国介入影像与治疗学,2021,18(1):23-27.
[14] VYAS V, PAUL M. Catastrophic complications following cryoablation of lung cancer [J]. Proc (Bayl Univ Med Cent), 2020, 34(1):131-132.
